Purpose and Background of our Research
Transforming growth factor-β (TGF-β) was identified in the early 1980s as a factor that induces the anchorage-independent growth of normal fibroblasts. TGF-β was subsequently found to inhibit the growth of most types of normal cells, including epithelial cells and lymphocytes. In the mid-1990s, TGF-β was shown to induce the epithelial-mesenchymal transition (EMT) in certain epithelial cells. TGF-β is now known to exhibit bi-directional effects, i.e. tumor-suppressive effects and tumor-promoting effects, during cancer progression (figure below).
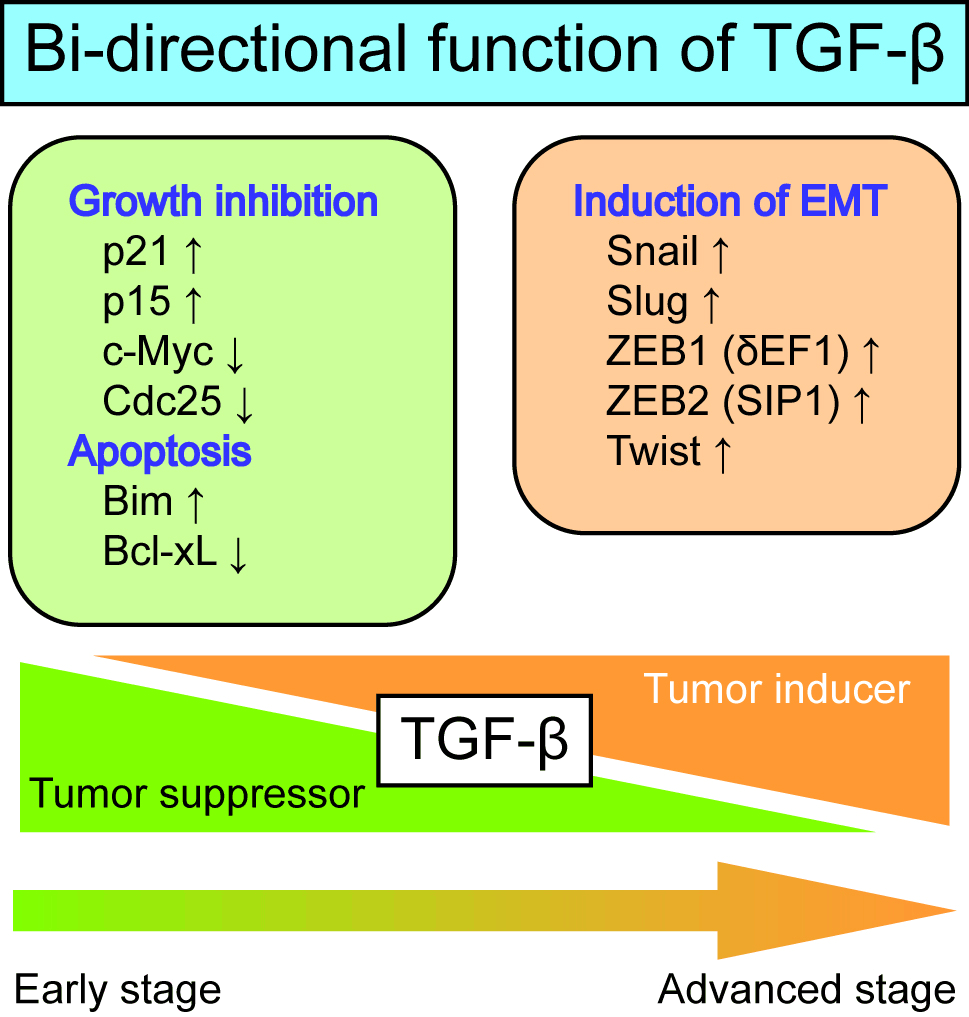
In our laboratory, we are investigating how TGF-β loses its tumor-suppressive effects and acquires tumor-promoting effects during cancer progression. We try to uncover the molecular mechanisms underlying the bi-directional effects of TGF-β on tumors using next-generation DNA sequencers, analyze EMT cell phenotypes using proteins and/or genes expressed in the cells undergoing EMT, and identify some TGF-β targets with therapeutic potential for certain types of cancer.
The biological importance and complicated mechanisms of action of TGF-β make them especially interesting to study. Much effort has been devoted to developing antagonists against TGF-β family proteins and their receptors, and some are in clinical trials. However, a better understanding of the TGF-β signaling pathways would facilitate the clinical development and application of such antagonists. The EMT is involved in the invasion and metastasis of cancer, but the underlying mechanisms are still not fully understood. Drugs that regulate the EMT have yet to be developed, and such drugs may be useful for treatment of certain types of cancer in the future.
(1) Investigate dynamic changes in the transcriptional machinery as regulated by the TGF-β-Smad pathways.
- Harada M, Morikawa M, Ozawa T, Kobayashi M, Tamura Y, Takahashi K, Tanabe M, Tada K, Seto Y, Miyazono K, Koinuma D. (2019). Palbociclib enhances activin-SMAD-induced cytostasis in estrogen receptor-positive breast cancer. Cancer Sci. 110(1):209-220.
- Arase M, Tamura Y, Kawasaki N, Isogaya K, Nakaki R, Mizutani A, Tsutsumi S, Aburatani H, Miyazono K, Koinuma D. (2017). Dynamics of chromatin accessibility during TGF-β-induced EMT of Ras-transformed mammary gland epithelial cells. Sci Rep. 7, 1166.
- Murai F, Koinuma D, Shinozaki-Ushiku A, Fukayama M, Miyaozono K, Ehata S. (2015). EZH2 promotes progression of small cell lung cancer by suppressing the TGF-β-Smad-ASCL1 pathway. Cell Discovery. 1, 15026.
- Isogaya K, Koinuma D, Tsutsumi S, Saito RA, Miyazawa K, Aburatani H, Miyazono K. (2014). A Smad3 and TTF-1/NKX2-1 complex regulates Smad4-independent gene expression. Cell Res. 24, 994-1008.
- Mizutani A, Koinuma D, Tsutsumi S, Kamimura N, Morikawa M, Suzuki HI, Imamura T, Miyazono K, Aburatani H. (2011). Cell type-specific target selection by combinatorial binding of Smad2/3 proteins and hepatocyte nuclear factor 4α in HepG2 cells. J Biol Chem. 286, 29848-60.
(2) Analyze cellular phenotypes regulated by the TGF-β-induced EMT, focusing the function of ZEB1 (δEF1) and other transcription factors.
- Katsura A, Tamura Y, Hokari S, Harada M, Morikawa M, Sakurai T, Takahashi K, Mizutani A, Nishida J, Yokoyama Y, Morishita Y, Murakami T, Ehata S, Miyazono K, Koinuma D. (2017). ZEB1-regulated inflammatory phenotype in breast cancer cells. Mol Oncol. 11(9):1241-1262.
- Mizutani A, Koinuma D, Seimiya H, Miyazono K. (2016). The Arkadia-ESRP2 axis suppresses tumor progression: analyses in clear-cell renal cell carcinoma. Oncogene. 35, 3514-23.
- Horiguchi K, Sakamoto K, Koinuma D, Semba K, Inoue A, Inoue S, Fujii H, Yamaguchi A, Miyazawa K, Miyazono K, Saitoh M. (2012). TGF-β drives epithelial-mesenchymal transition through δEF1-mediated downregulation of ESRP. Oncogene. 31, 3190-201.
- Shirakihara T, Horiguchi K, Miyazawa K, Ehata S, Shibata T, Morita I, Miyazono K, Saitoh M. (2011). TGF-β regulates isoform switching of FGF receptors and epithelial-mesenchymal transition. EMBO J. 30, 783-95.
(3) Determine the mechanisms of cancer invasion and metastasis induced by the TGF-β through analyzing new Smad2/3 target genes identified by RNA sequencing and other methods.
- Nishida J, Miyazono K, Ehata S. (2018). Decreased TGFBR3/betaglycan expression enhances the metastatic abilities of renal cell carcinoma cells through TGF-β-dependent and -independent mechanisms. Oncogene. 37(16):2197-2212.
- Kawasaki N, Isogaya K, Dan S, Yamori T, Takano H, Yao R, Morishita Y, Taguchi L, Morikawa M, Heldin CH, Noda T, Ehata S, Miyazono K, Koinuma D. (2018). TUFT1 interacts with RABGAP1 and regulates mTORC1 signaling. Cell Discov. 9;4:1.
- Sakurai T, Isogaya K, Sakai S, Morikawa M, Morishita Y, Ehata S, Miyazono K, Koinuma D. (2016). RNA-binding motif protein 47 inhibits Nrf2 activity to suppress tumor growth in lung adenocarcinoma. Oncogene. 35, 5000-9.
- Hoshino Y, Nishida J, Katsuno Y, Koinuma D, Aoki T, Kokudo N, Miyazono K, Ehata S. (2015). Smad4 Decreases the Population of Pancreatic Cancer-Initiating Cells through Transcriptional Repression of ALDH1A1. Am J Pathol. 185, 1457-70.
- Katsuno Y, Ehata S, Yashiro M, Yanagihara K, Hirakawa K, Miyazono K. (2012). Coordinated expression of REG4 and aldehyde dehydrogenase 1 regulating tumourigenic capacity of diffuse-type gastric carcinoma-initiating cells is inhibited by TGF-β. J Pathol. 228, 391-404.
- Ehata S, Johansson E, Katayama R, Koike S, Watanabe A, Hoshino Y, Katsuno Y, Komuro A, Koinuma D, Kano MR, Yashiro M, Hirakawa K, Aburatani H, Fujita N, Miyazono K. (2011). Transforming growth factor-β decreases the cancer-initiating cell population within diffuse-type gastric carcinoma cells. Oncogene. 30, 1693-705.
